Statistical physics
The substance Statistical
Physics microstructure and microscopic particles understanding the interaction with the statistical probability of the method , making interpretation of microscopic physical properties of the
macroscopic object consisting of a large number of particles and the
orderliness of theoretical physics branch. Also known as statistical mechanics . The so-called large amount is
based on the number of molecules contained in a mole of material
Research object
The research object
changes from a small number of individuals to a group composed of a large
number of individuals, resulting in fundamental changes in the nature of the
law and research methods. The statistical laws followed
by a large number of particle systems cannot be reduced to mechanical laws. Statistical
Physics by microscopic to
macroscopic bridge, which provide the basis for a variety of macroscopic
theory, has become a gas, liquid, solid, and plasma basic theory body, and play a role
in the study of biology and chemistry. Gas kinetic theory (formerly
known as gas molecule movement theory )
is an early statistical theory. It reveals the microscopic nature of macroscopic quantities such
as gas pressure ,
temperature, and internal energy , and gives the relationship between them
and the corresponding average of the microscopic quantities . The
derivation of the average free path formula,
the establishment of the gas molecule velocity or velocity distribution law,
the energy sharing theorem, and the relevant data are obtained, so that people
can balance the thermal motion ,
collision, and energy distribution of
the ideal gas molecule
in equilibrium . With
a clear physical image and quantitative understanding, it also shows the special
importance of probability and statistical distribution to
statistical theory.
Theoretical basis
The establishment of the
non- equilibrium distribution function and its evolution equations not only become the basis of the
micro-statistic theory of the transportation process, but also the H function defined by
it and the H theorem that it follows to understand, the
irreversibility of the macro process and the process tending to equilibrium
Played an important role. Elucidation of the statistical significance of entropy and microscopic statistical interpretation of the principle of entropy
increase indicate that statistical theory has evolved from
equilibrium to non-equilibrium, and has evolved from microscopic statistical
interpretation of certain macro concepts and macro laws to the second law of thermodynamics Such
general laws make microscopic statistical interpretations. However, the theory of gas kinetics uses molecules as statistical entities,
and it is necessary to make unfounded guesses or assumptions about the
structure of molecules and the interaction between molecules. This is the
fundamental difficulty and limitation for its further development.
Research
method
JW Gibbs took the
entire system as a statistical entity, and proposed to study the
distribution of ensemble composed of a large number of systems in
the phase space , which overcomes
the difficulty of gas kinetic theory and establishes statistical
physics. In the statistical theory of equilibrium
state , the micro-regular ensemble is used for the isolated system with fixed energy and particle number ;
for the system that can exchange energy with the large heat
source but the particle number is fixed, the regular ensemble is used ; for the system that can exchange energy with the large heat
source and The particle system uses giant regular ensemble . These are three commonly
used systems, and the distribution density function of each ensemble in the
phase space has been obtained. Quantum statistics and classic statistics have
the same research objects and research methods , and the concept of ensemble is still
applicable in quantum statistics. Quantum Statistical difference is
that microscopic particle motion follows the mechanics of quantum rule rather than mechanical classic law of microscopic motion state having a discontinuity , required quantum state rather
than to buildings described.
Non- equilibrium statistical
physics has a wide range of contents, and it is a subject that is still
developing rapidly and is far from mature. The system is near equilibrium,
which tends to study equilibrium relaxation time and temperature dependencies; do not too
far away from equilibrium, maintaining the temperature difference,
concentration difference, the potential
difference and the like various transport process system
undergo , To study its various linear transport
coefficients, in addition, to study fluctuations . Relaxation ,
transportation, and fluctuation are the main non-equilibrium processes near the
equilibrium state.
Historical origin
Since the 1960s, to far from equilibrium of physical phenomena have been extensively studied, the most important thing
is far from equilibrium mutation, ordered structures arise, the establishment of a dissipative structure theory , but has not yet formed a complete
theoretical system






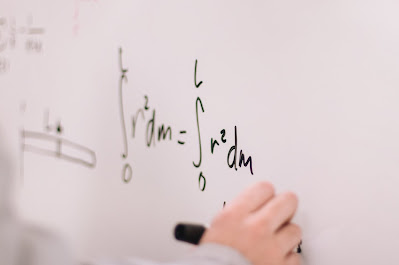





Comments
Post a Comment